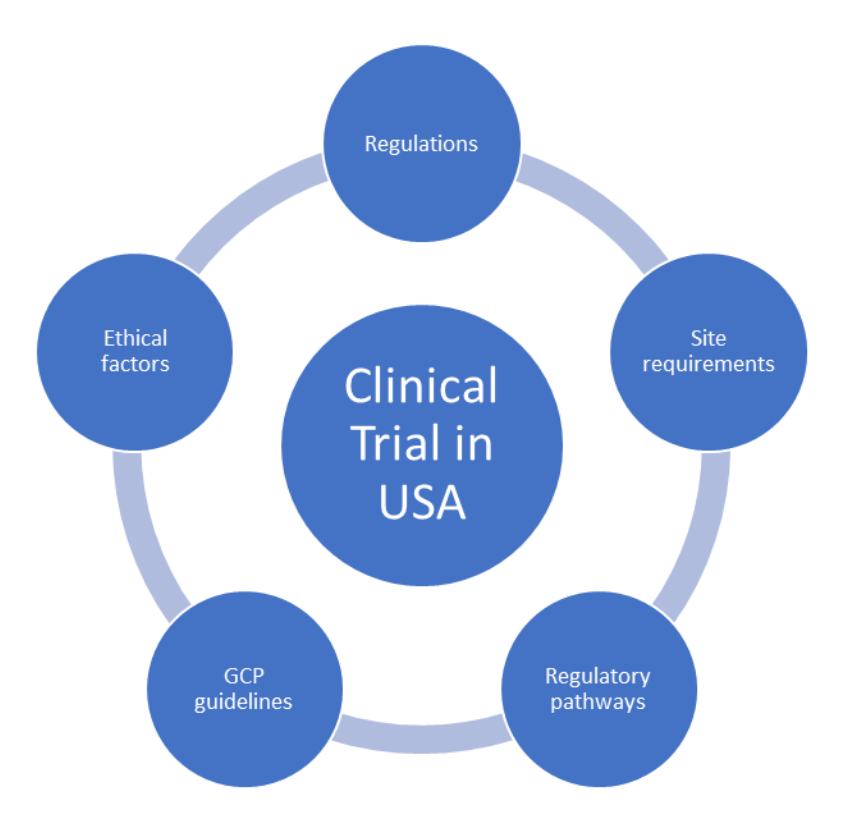
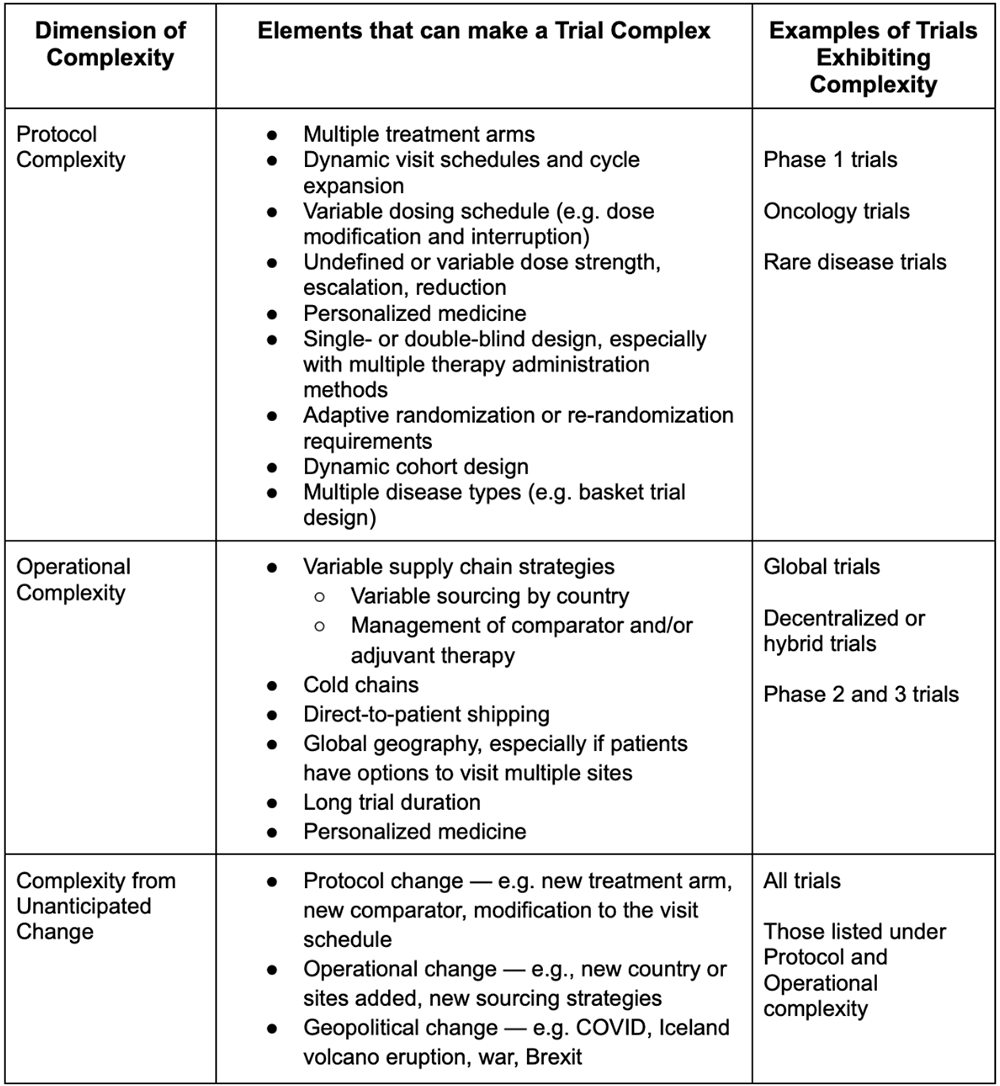
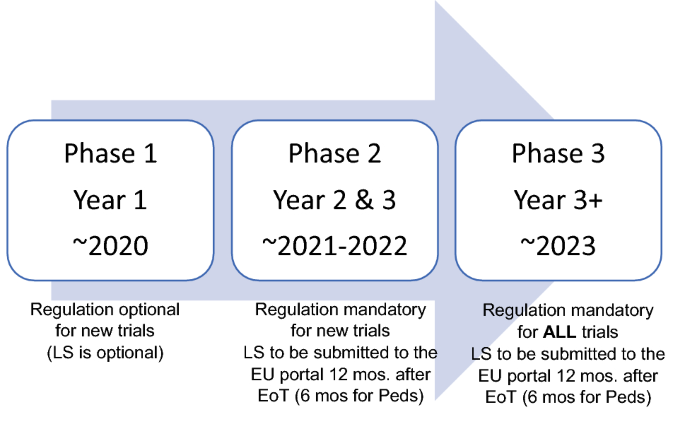
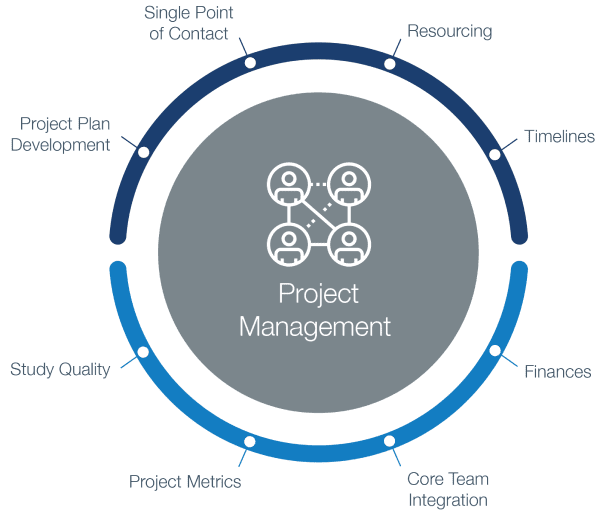
ADVANCED CLINICAL TRIALS THE CLINICAL TRIAL PROCESS: IMPENDING CHANGES IN THE REGULATORY FRAMEWORK - ADVANCED CLINICAL TRIALS
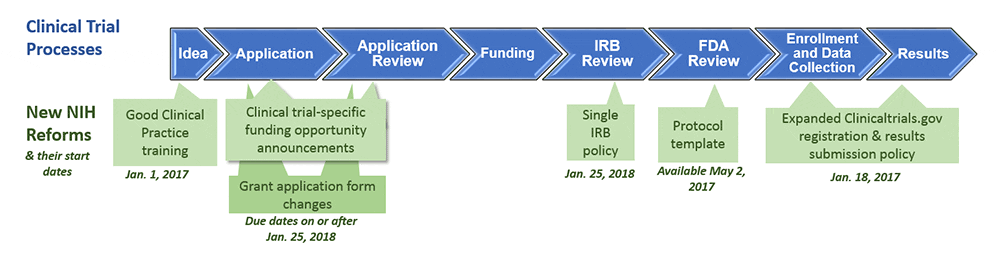
NIH Redefines Clinical Trials and Sets New Requirements: Is Your Human Subjects Research Affected? - ASHG
![PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/52893bcfc57fecda0c60e34dd1fe46414c867575/9-Figure4-1.png)
PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar